
Lecture Notes #17 Amino Acid Metabolism
Introduction - amino acids which result from the hydrolysis of proteins may also enter cellular respiration, but must first undergo a deamination reaction.
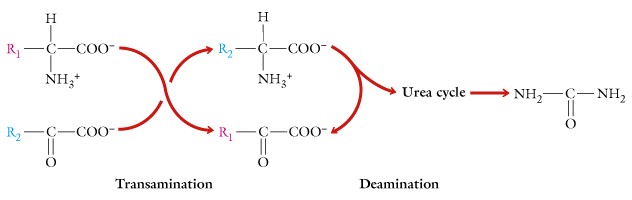
There are several methods for removing the amino functional group and a major one is termed transamination.
In this process the amino group is transferred to alpha-ketoglutaric acid to form glutamate, which can be transported into mitochondria of the liver where it is oxidatively deaminated with the enzyme glutamate dehydrogenase and NAD. The ammonium ion formed then enters the urea cycle which is coupled to mitochondria in a manner termed the "Krebs Bicycle"and via a series of reactions between the cytosol and the mitochondria which results in the formation of urea. Urea contains a carbonyl functional group and two amino groups. The urea is non-toxic and soluble in water and ready for secretion. The urea cycle requires energy in the form of ATP and about 15% of the energy content of an amino acid is required for this synthesis.
Review the urea cycle in the text book and be sure you understand the process.
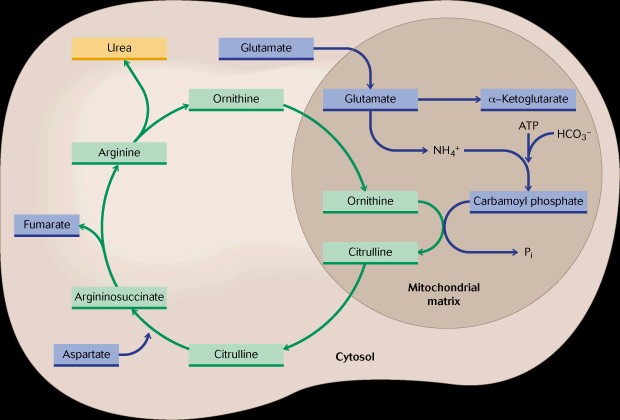
Note that in mitochondria the ammonium ion is combined with bicarbonate and phosphate to form carbamoyl phosphate and that this is the initial step in urea biosynthesis.
In addition to transamination, oxidative deamination using the coenzyme NAD can be used to produce ammonia.
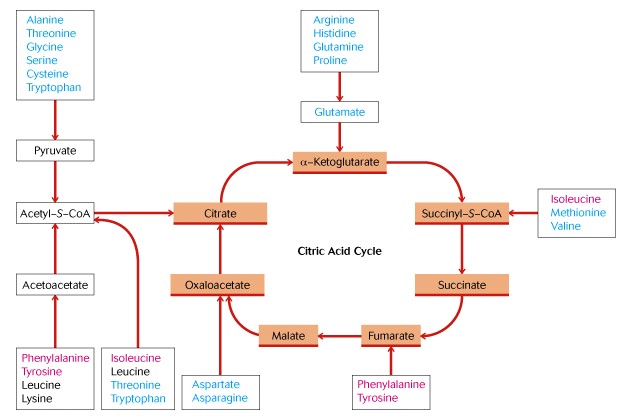
Note the carbon skeletons of Amino Acids enter energy metabolism at their appropriate place:
This is the reverse of reductive amination used in the amination of carbon skeletons to form amino acids. The major toxic effect of ammonia is its influence on cellular pH and the depletion of TCA intermediates.
Recall that the ammonium ion is a weak acid and upon ionization it produces a proton and ammonia (NH3) which is a strong base.
This is another example of the reverse relationship whereby strong acids produce weak conjugate bases, and weak acids in turn produce strong bases.